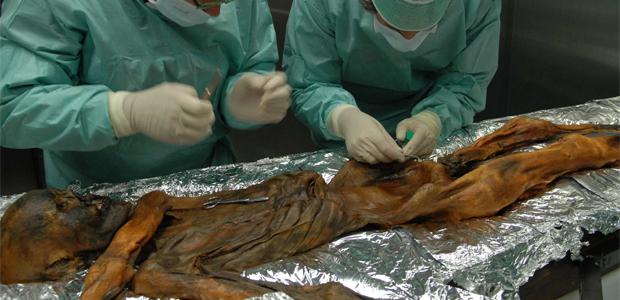
An international team of researchers has sequenced the entire genome of the Tyrolean Iceman, uncovering a plethora of information about his ancestry, identity and his health – he’s even stepped into record books as the oldest documented case of lyme disease in humans.
Since he was discovered in 1991, researchers from around the world have scanned, poked and sampled the 5,300 year-old mummified-man, known as Ötzi.
Now, an international team of researchers have sequenced the entire genome of the Tyrolean Iceman, uncovering a plethora of information about his ancestry, identity and his health – he’s even stepped into record books as the oldest documented case of lyme disease in humans.
“We found that he belongs to a Y-haplogroup that originated in the near east and spread over Europe,” Albert Zink, study co-author and head of the Institute for Mummies and the Iceman in Bolzano, Italy told Science-Fare.com.
The Y-haplogroup traces the heritage through Ötzi’s father’s side. His Y-haplogroup, G2a4-L91 has largely settled on islands in the Tyrrhenian Sea, just west of Italy, and was essentially diluted on the mainland populations as more groups moved across and settled in Europe.
“It remained in a high percentage, especially in Sardinia and Corscia,” Zink said. “It means the Sardinians and the Iceman have the same ancestor.” The same can be said about the people of Corsica.
Previously, a sequence of Ötzi’s genome that traces the heritage of his mother, called mitochondrial DNA – which is housed in the cell’s energy factory – found Ötzi to be part of a new and rare mitochondria- haplogroup, called K1.
When researchers compared that sequence data to theirs, they found a near-perfect match.
“It’s important to have these controls,” Zink said. “The big issue with analyzing ancient DNA is that you can have sample contamination.” Because they got the same unique result from a different sample, they knew it was Ötzi they were sequencing and not someone previously in contact with him.
Part of what makes Ötzi so fascinating to researchers is his age – they’ve even examined the contents of his gut. Europe was in a major transition from the Neolithic age to the Copper one when Ötzi roamed the Alps and the general diet was beginning to change.
In addition to showing genetic signs of the pathogen known to cause Lyme disease – qualifying as the oldest documented case of that disease – one of the more salacious discoveries was that Ötzi’s lactose intolerant. Specifically, he carried what’s now the recessive version of the MCM6 gene.
In addition to helping the cell divide properly – by helping unzip the DNA so it can replicate – a single base-change in the DNA is responsible for promoting the continuous generation of the protein lactase, the enzyme that breaks down lactose in milk.
“This is somewhat to be expected,” Zink said. “In this time period, they started domesticating animals and the main population was intolerant.”
Previous research by other groups failed to find this change in DNA from samples that are even older than Ötzi’s and both his copies of MCM6 show the recessive form – compared to today’s population.
It lends more support to the theory that it was the availability of milk to the general public – through large scale farming – that allowed those with the mutation to thrive.
Those without the change gradually see the gene that makes lactase turn off and that’s the general basis for lactose intolerance as we know it today.
The general consensus in the scientific community is that Ötzi died from an arrow wound. If he would have survived, researchers say his own genetics may not have been much more kind
“He had predisposition for coronary heart disease – a very strong one,” Zink said. “He was in a high risk group to suffer a stroke or similar event in the following years.”
They found Ötzi to harbour changes in at least six different genes associated with Coronary Heart Diseases, like strokes and heart attacks. Considering just two of the genes, his risk of suffering an event doubled from as much as 40 per cent to as high as 80 per cent.
“We also found evidence from a previous computer tomography investigation that he had already suffered from some atherosclerosis,” Zink said.
From that investigation, radiologists found calcium build-up in Ötzi’s carotid and right iliac arteries, as well as in his distal aorta. The six risk factors explain why those calcifications are due to bad genes as much – if not more than – his diet or level of fitness.
Not to outdo themselves, researcher also generated an image of Ötzi that showcases his newly discovered brown eyes – all thanks to a sample removed from his hip bone.
They also found he was blood type O and expressed Rh-positive antigens. The Rhesus factor’s the second most important one considered when matching donated blood to needing recipients – second only to the blood group itself.
Ötzi’s genome is just one of many that have been sequenced using next generation sequencing technology. Thanks to incredible advances, a genome that used to cost millions of dollars and take countless hours to sequence can now be done in a fraction of the time and for a sliver of the price.
“It took us maybe three-to-four months to get all the sequencing data,” Zink said. It took about a year after that for the group to finish the preliminary analysis.
The technology also makes it easier to work with and process delicate samples, like Ötzi’s.
“After the death of an individual the DNA degrades very rapidly,” Zink said. “The big advantage of the iceman was that he was kept in the cold for 5,300 years, which is positive for DNA preservation.”
Spontaneous deamination is one of those processes and like its name suggests, it’s the spontaneous change in an individual DNA base that changes how it’s read and interpreted during sequencing.
Having more accurate technology helps researchers distinguish between the true genome of Ötzi and the areas of his DNA that have changed as his DNA degraded – thankfully for researchers, at a substantially slower rate that can be studied.
The research was published in the latest issue of the journal, Nature Communications.
 Science Fare Media Science News – Upgraded
Science Fare Media Science News – Upgraded